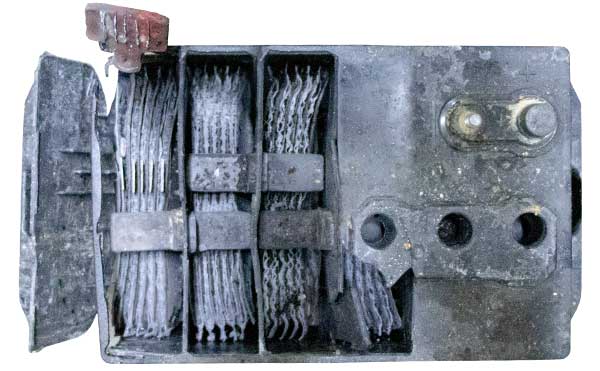
Battery Sulfation
Although sulfation is a normal part of the chemical reaction within a lead-acid battery, it is responsible for over 80% of all lead-acid battery failures. Normal levels of sulfation are broken down during regular charging cycles. However, when it builds-up and larger crystals form that affix themselves to the lead plates, they rob battery capacity. The battery loses its ability to fully charge which decreases equipment run time and increases labor costs. At this stage, the sulfate crystals cannot be broken down by charging alone.